Written By: Kaitlyn Sadtler
Original Article: Davidson et al. Acta Biomaterialia 2020
The Gist of It:
Just like we take in information from our environment and change our behavior (like putting a coat on when it’s cold outside), cells take in information from their environment – the extracellular matrix. The cues they receive can cause them to change their environment by secreting different proteins, making the matrix stiffer or softer (just like we can turn on the A/C or put another log on the fire to make it cooler or warmer). Stiffening and densifying of the extracellular matrix is called fibrosis, and this process contributes to numerous disorders, including liver cirrhosis, cardiac dysfunction, skin scarring, and lung fibrosis. Understanding how our cells sense changes in the stiffness of the matrix around them and how that affects disease progression is necessary for the development of new therapeutics. Researchers from the University of Michigan Ann Arbor have developed a new material and platform to study this phenomenon in the laboratory. Davidson and colleagues synthesized a fibrous biologic matrix out of dextran vinyl sulfone (DexVS). The researchers were able to spin these fibers onto a plate to create a sort of cellular hammock, where the cells would feel just the stiffness of the material and not the plastic bottom of a petri dish. With these fibers, they were able to look in a highly controllable manner at how cells interacted with stiffer and softer matrices. In contrast to what had been seen when the interaction between cells and the matrix was studied using other methods, like looking at solid synthetic gels (jello-like), they found that stiffer materials made cells less fibrotic than softer materials did. Using these models, scientists will be able to probe interactions between cells and fiber-based matrices that are more similar to the natural extracellular matrix and compare to previous studies which used fully synthetic hydrogels that do not have fibers. Moving forward, these scientists have developed ways to modify these materials with different proteins and change the way that the cells interact with the matrix. Further research like this could expand our understanding of what happens to our cells in stiff environments, which could lead to therapeutics for fibrotic diseases.
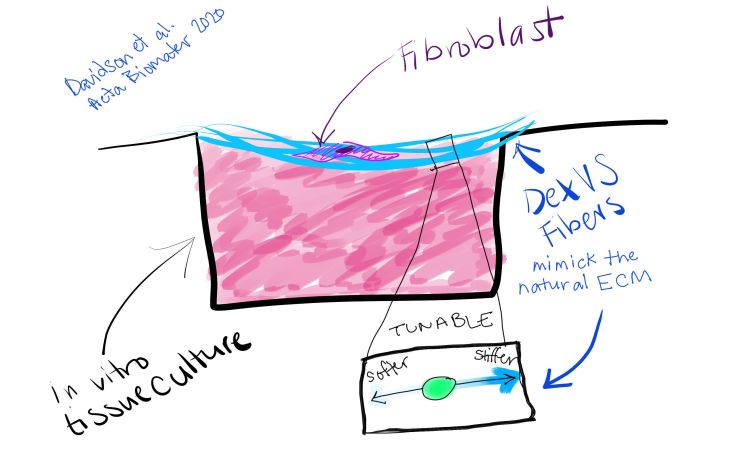
New Dextran Vinyl Sulfone (DexVS) fibers are tunable, which means scientists can alter the stiffness of each fiber, along with the stiffness of the overall synthetic extracellular matrix they have created. Using a draped matrix, cells in contact with the fibers will only feel stiffness of those fibers, and not the plastic around them.
The Nitty Gritty:
Previously, researchers had developed an electrospun methacrylated dextran (DexMA) to study matrix stiffness in vivo. However, DexMA fibers degraded in vitro due to hydrolytic cleavage of the ester bonds in the polymeric matrix within days of exposure to cell culture media. Here, the researchers presented a dextran vinyl sulfone (DexVS) that lacks the sensitive ester bonds and can still be functionalized via Michael-type addition and crosslinked with a photoinitiator (in this case, lithium phenyl-2,4,6-trimethylbenzoylphosphinate, LAP). They tested the stiffness of these materials by changing the concentrations of photoinitiator or duration of UV exposure. They further functionalized these materials with cyclic RGD (cRGD) for cell attachment or with methacrylated heparin to promote association of cell-secreted matrix with the synthetic polymer fibers. DexVS fibers were spun onto a multi-well plate to create a suspended surface with mechanical properties independent of the tissue culture plastic. Culturing normal human lung fibroblasts (NHLFs) on stiffer and softer matrices revealed that softer fibrous matrices, in the presence of pro-fibrotic soluble transforming growth factor beta-1 (TGFβ1), induced higher levels of myofibroblast-associated alpha smooth muscle actin (αSMA) than did stiffer matrices. This is contradictory to studies performed with solid hydrogels, such as poly(ethylene glycol) (PEG)-based hydrogels, that suggested stiffer matrices induce more of the fibrotic myofibroblast phenotype. Further research using multiple model systems in vitro and comparison to in vivo samples will hone in vitro modeling to pave the way for understanding mechanistic biology, modeling disease, and screening novel therapeutics.